| Table of Contents |  |
|
Original Article
| ||||||
| Improvement in the inner ear symptoms of patients with Meniere's disease after treatment using low-frequency vibration: A preliminary report | ||||||
| Jing Zou1, Rishunzi Peng1, Guiliang Zheng1, Qing Zhang1, Esko Toppila2, Hongliang Zheng3, Ilmari Pyykkö4 | ||||||
|
1Department of Otolaryngology-Head and Neck Surgery, Center for Otolaryngology-Head & Neck Surgery of Chinese PLA, Changhai Hospital, Second Military Medical University, Shanghai, China. Hearing and Balance Research Unit, Field of Otolaryngology, School of Medicine, University of Tampere, Tampere, Finland.
2Hearing and Balance Research Unit, Field of Otolaryngology, School of Medicine, University of Tampere, Tampere, Finland. 3Department of Otolaryngology-Head and Neck Surgery, Center for Otolaryngology-Head & Neck Surgery of Chinese PLA, Changhai Hospital, Second Military Medical University, Shanghai, China. 4Hearing and Balance Research Unit, Field of Otolaryngology, School of Medicine, University of Tampere, Tampere, Finland. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Zou J, Peng R, Zheng G, Zhang Q, Toppila E, Zheng H, Pyykkö I. Improvement in the inner ear symptoms of patients with Meniere's disease after treatment using low-frequency vibration: A preliminary report. Edorium J Otolaryngol 2015;2:5–13. |
|
Abstract
|
|
Aims:
To evaluate the efficacy of controlling the inner ear symptoms for the duration of 7 – 19 months specifically the vertigo in patients with Meniere's disease (MD) using a novel therapy that delivers low-frequency vibrations to the inner ear through the mastoid process.
Methods: The system is composed of vibrational power generator that was driven by the centrifugal force of the rotating weight, and a head holder that couples the vibrator to the temporal bone skin. 14 MD patients were exposed for 30 min to vibrations at a fundamental frequency of 100 Hz at an intensity of 70.6 m/s2 and consisting of a cluster of harmonic waves ranging from 148 to 1120 Hz. The cervical vestibular evoked myogenic potentials (cVEMPs) were measured in the MD patients before and at 30 min post-vibration treatment. Gadolinium-enhanced MRI scan was used to detect potential endolymphatic hydrops in MD patients. The complaints in MD patients were followed for 7–19 months. Results: Twelve out of 14 patients felt comfortable within 24 h after exposure to the low-frequency vibration except that 2 patients did not show any benefit. The vertigo were significantly controlled by the novel therapy (p<0.01, Wilcoxon signed ranks test). There was insignificant improvement in hearing function after the treatment. The tinnitus and ear fullness were significantly improved after exposure to the low-frequency vibration. The side difference in the amplitudes of cVEMP (contralateral side minus ipsilateral side) became smaller at 30 min posr-vibration than that before vibration (p<0.05, Wilcoxon signed ranks test). Conclusion: The novel low-frequency vibration therapy was effective in controlling vertigo, tinnitus, and ear fullness of patients with MD. | |
|
Keywords:
Vibration, Meniere's disease, Vertigo, Therapy, Low-frequency
| |
|
Introduction
| ||||||
|
Meniere's disease (MD) is a chronic, fluctuant disorder arising from malfunction of the inner ear affecting up to 0.5 % of the population [1] [2]. The diagnosis of MD is based on a symptom entity of at least two rotatory vertigo attacks each lasting at least 20 min, tinnitus, and documented hearing loss [3]. The etiology of the condition is unknown and the course is non-predictable leading to functional limitations [4]. MD significantly affects patients' quality of life, by restricting activities and participation [5] [6]. The most distinctive pathological feature of MD is endolymphatic hydrops [7], which was originally observed in histopathological studies and has recently been visualized using gadolinium-enhanced MRI scan [8] [9]. Based on this histopathology, Portmann proposed endolymphatic-sac surgery as a therapy for MD in 1923 [10] . However, Thomsen et al. challenged the benefits of endolymphatic-sac surgery in MD by comparing the efficacy of an endolymphatic-sac mastoid-shunt operation with a mastoidectomy among patients with typical MD [11]. They observed 70% improvement in both groups, and interpreted that the outcome was due to placebo effect. However, the patients' improved conditions remained stable for at least 9 years [12]. The endolymphatic sac surgery relieves vertigo in most patients, but the mechanism of such symptomatic relief remains unknown. Chung et al. reported histopathological analysis of the temporal bones in 15 patients with MD who had passed endolymphatic sac surgery and concluded that the surgery did not relieve the endolymphatic hydrops [13]. These studies suggested that the successful control of vertigo was not due to the successful placement of the shunt within the endolymphatic sac. Instead, the beneficial results may come from shear stress related phenomenon caused by drilling into the temporal bone of the MD patients [14] [15] [16] . During the performance of both mastoidectomy and endolymphatic-shunt surgery, the burr generates significant vibration to the inner ear resulting in shear stress of the vestibular system which is similar to the vibration effect seen in the cochlea that also seems to cause hearing loss [14] [15] [17]. We hypothesized that the burr-induced vibration modified the vestibular activity of MD patients and re-balanced the neural excitability on both sides. Shear stress in the cochlea was first reported by Zou et al. in 2005 in an animal model of vibration-induced hearing loss [15]. However, the results obtained in that study cannot be translated to treatment of vertigo because the system generated impulses with high levels of noise up to 114 dB SPL due to loose coupling of the electromagnetic shaker with skull. The vibration occurred at higher frequencies that contributed to hearing loss [17]. We further hypothesized that the low-frequency vibration can be useful in treating MD patients and developed a device that is capable of generating low frequency vibrations with high amplitude to guarantee stimulating the vestibular system with low-frequency and low intense noise that is not very hazardous to the auditory system (CN103230646A) [14] [16][18]. There are several reports on the effect of vibration on vestibular response in both patients and animal studies. Vibration of the mastoid tip on the neck activated among others the neck muscles and led to body sway that could be recorded on posturography [19]. The induced reflex by this kind of vibration is organized by the vestibular system through vestibule-spinal pathways. It is exaggerated among patients with vestibular lesion or brain stem lesions [20]. In patients with unilateral vestibular lesions, Lucke detected nystagmus induced by bone vibration on the skull [21] . Dumas et al. conducted videonystagmography of patients with various vestibular diseases and showed that the frequency that elicited the strongest nystagmus was 100 Hz [22]. Manzari et al. reported ocular and cervical vestibular-evoked myogenic potentials (oVEMPs/cVEMPs) for bone-conducted vibration in MD patients during symptom-free intervals vs acute attacks, and found that during MD attacks the dynamic utricular function in the affected ear (as measured by the n10 wave of the oVEMPs at 500 Hz) was enhanced whereas the dynamic saccular function in the affected ear (as measured by the p13 of the cVEMP to 500 Hz bone-conducted vibration) was reduced [23]. These findings suggested that the vestibular system was potentially modulated by the low-frequency vibration. However, there are no reports on treating MD using any type of vibratory system. Our novel device for delivering low-frequency vibration in treating MD patients through the mechanism of shear stress was patented recently (CN103230646A). The novel system mimicked the effect of the drilling performed during endolymphatic-sac surgery but avoided the high frequency components. The system was shown to be capable of stimulating the vestibular system and safe for the auditory system (data will be reported separately). The aim of this study was to observe the response of MD patients who received treatment using the novel system. | ||||||
|
Materials and Methods
| ||||||
|
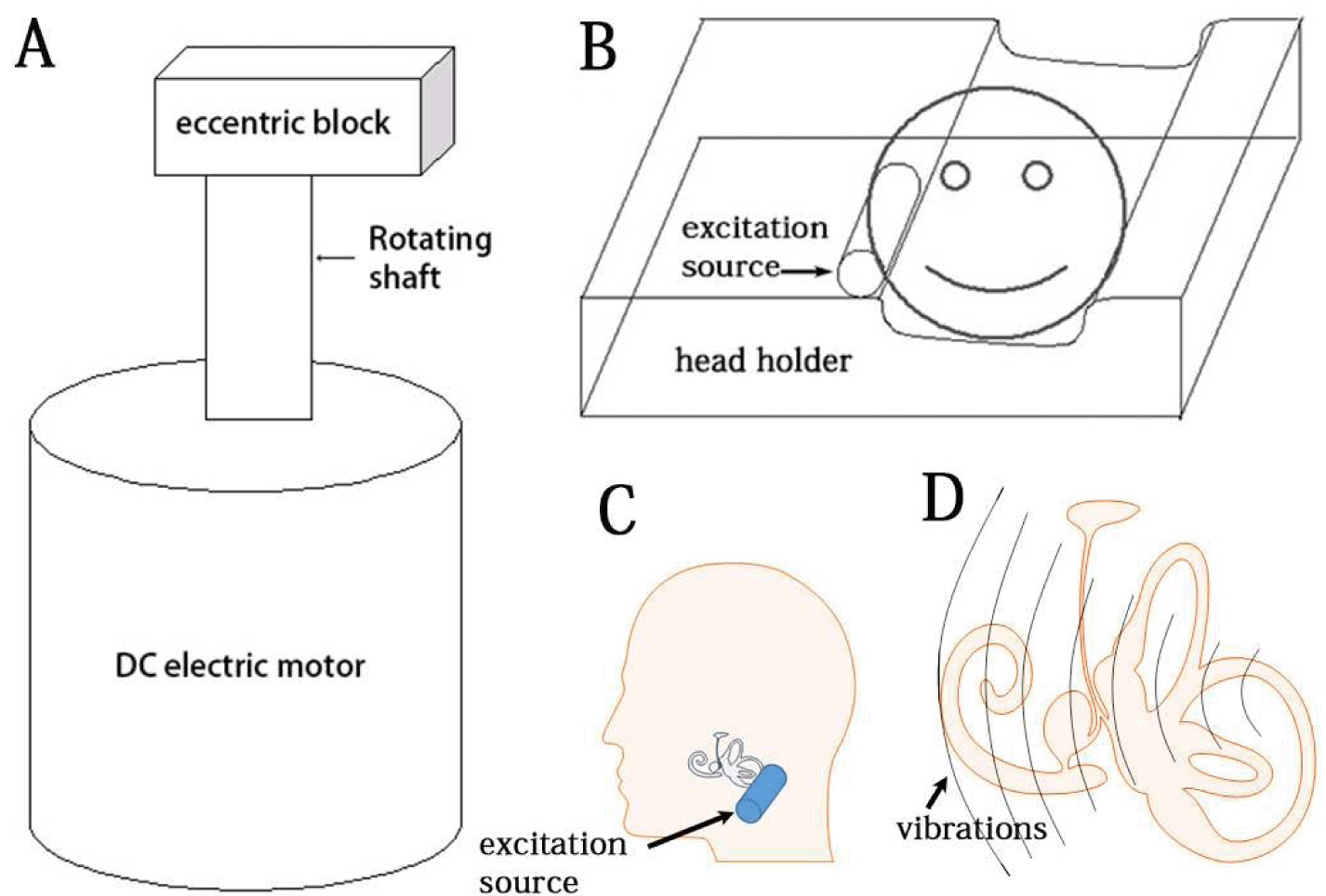
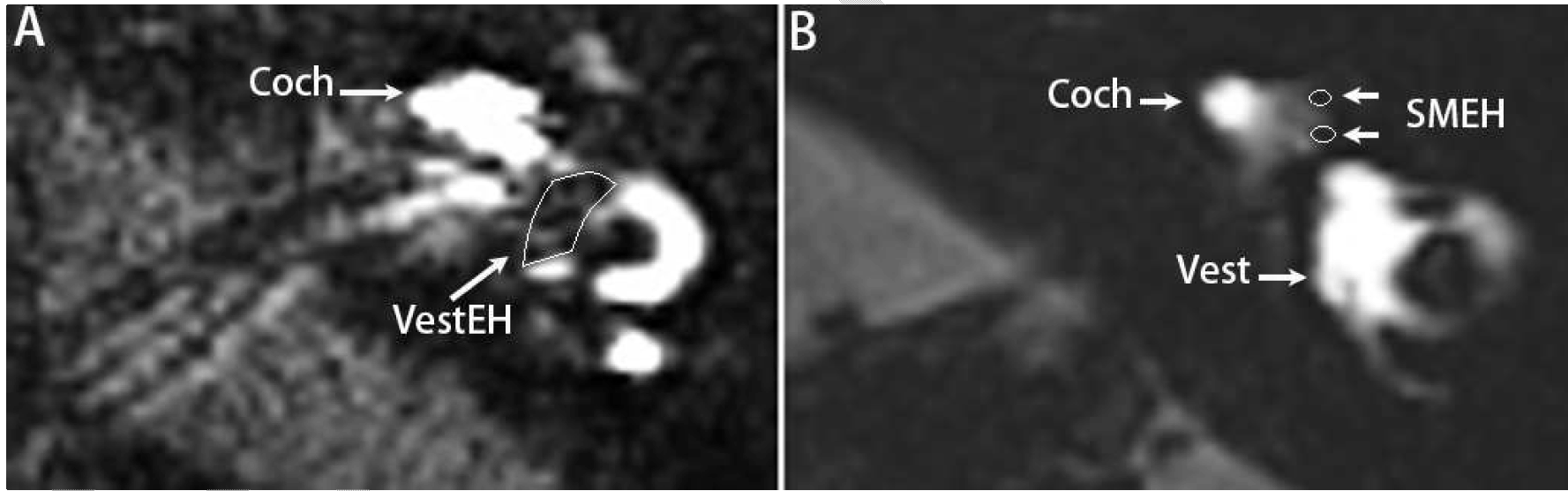
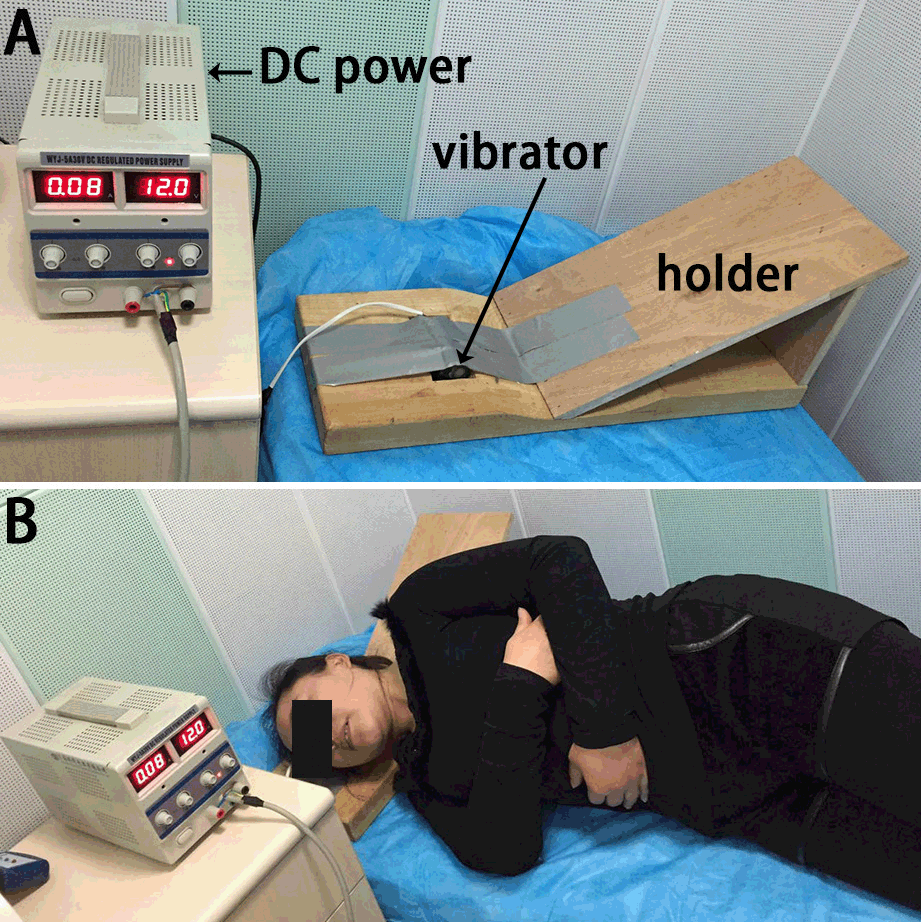
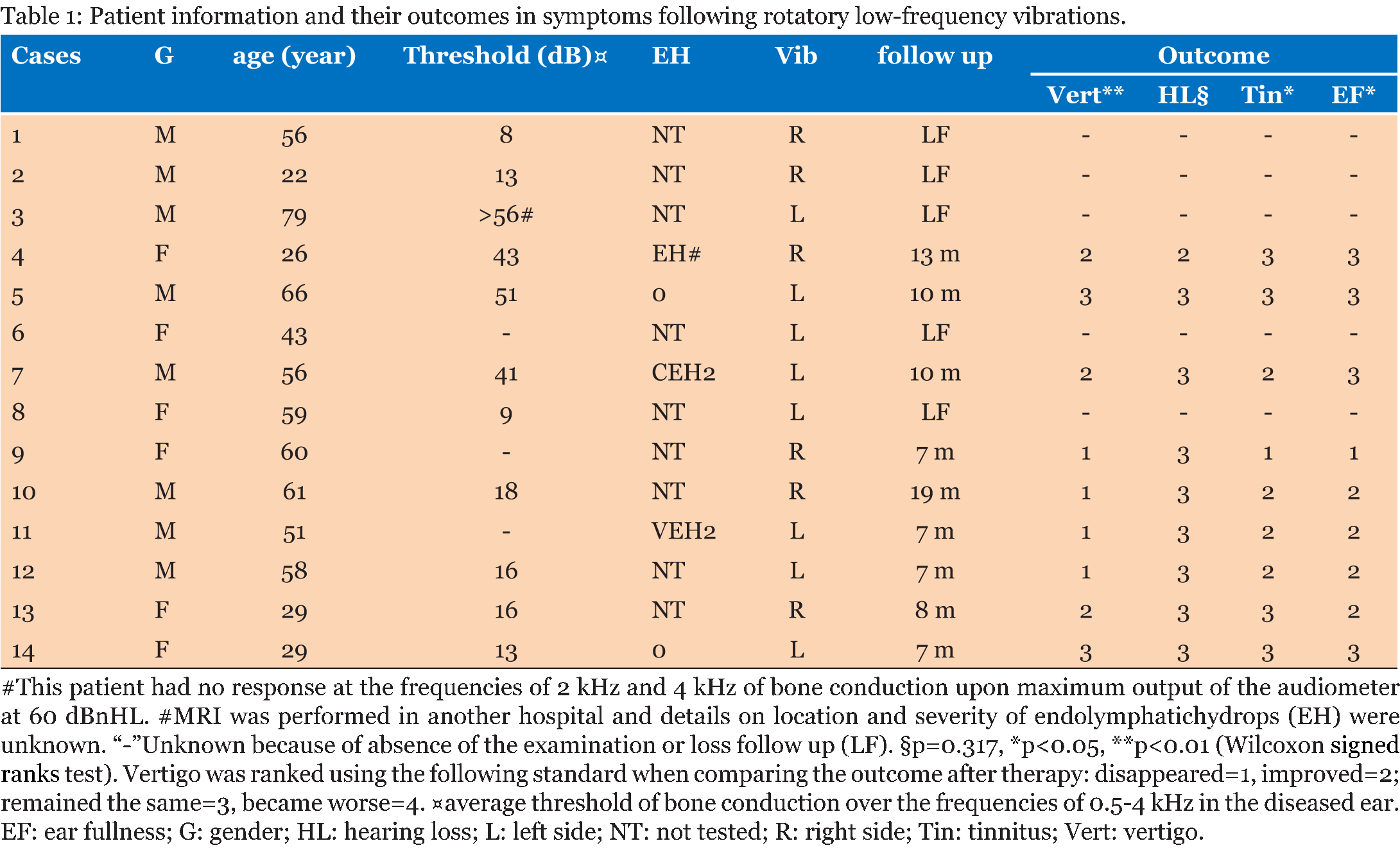
Design of the low-frequency vibration-delivery system: The novel low-frequency vibration-delivery system for vertigo therapy consisted of an excitation source that was a combination of a power source and a vibrator, in which an eccentric weight was installed on the rotating shaft of the direct current electric motor. The system generated vibration caused by centrifugal force of the rotating weight (Figure 1A), and was held in contact of temporal bone skin with a holder that delivered the vibration to the temporal bone (Figure 1B and 1C) and to the vestibular end organ (Figure 1D) (CN103230646A). Participants: Fourteen unilateral MD patients, who visited the outpatient Department of Otolaryngology-Head and Neck Surgery of the Changhai Hospital affiliated with the Second Military Medical University between May 2013 and March 2014, were enrolled in the study as volunteers. All of the patients were diagnosed as having definitive MD according to the guidelines of the AAN-HNS Committee of Hearing and Equilibrium that were published in 1995 [3]. They were not responsive to routine therapies including the administration of betahistine dihydrochloride, flunarizine hydrochloride, prednisone acetate, and mecobalamin. The following clinical data are presented in Table 1: patient's age, gender, symptomatic time course, diagnosis, MRI evaluation of endolymphatic hydrops, vibration treatment, and follow-up time. The degree of endolymphatic hydrops in the vestibule was graded according to the method of Nakashima et al. [24]. The site of endolymphatic hydrops in the cochlea was gradated as follows: 0=not detected, 1=apical endolymphatic hydrops, and 2=basal turn endolymphatic hydrops. The role of apical endolymphatic hydrops in MD is uncertain [25]. Typical endolymphatic hydrops in the vestibule of patient 11 and in the cochlea of patient 7 were shown using MRI at 12 h post-transtympanic injection of gadopentetate dimeglumine (Gd-DTPA) (Figure 2). The protocol was reviewed and approved by the ethical committee of The Sixth People's Hospital affiliated with Shanghai Jiaotong University, China (permission number 2013–18). All the subjects were recruited on a voluntary basis with their signature on the informed consent form. All of the protocols followed the rules of the Declaration of Helsinki, which was developed by the World Medical Association and was updated at the 64th WMA General Assembly in Fortaleza, Brazil, in 2013 [26]. Delivery of low-frequency vibration to the mastoid process of volunteers: To deliver the vibration for mastoid area, the volunteers were investigated in a lateral position with the contralateral ear upward (Figure 3). This set up generated vibration at fundamental frequency of 100 Hz at an intensity of 70.6 (m/s2) with a cluster of harmonic waves ranging from 148 to 1120 Hz (the intensity of the harmonics was 1E+5 to 1E+7-folds lower than that of the fundamental frequency); the total exposure time was 30 min. The vibration delivery was performed once for the 14 patients with MD and was repeated for three of the patients. Among the patients with repeated exposure, 1 patient did not benefit from the previous vibration and 2 patients desired a repeat expecting to strengthen the effective results. Measurement of cVEMPs: The cVEMPs were recorded using an ICS CHARTR EP Auditory Evoked Potential System (GN Otometrics, Denmark). After the skin was prepared, the active surface electrode was placed over the superior-middle third junctional part of the contralateral sternocleidomastoid muscle, the reference electrodes were placed on the superior aspect of the sternum, and the ground electrode was placed on the forehead. Air-conducted alternating 500-Hz tone bursts (95 dB nHL, Hanning envelope, duration of 5 ms) were presented unilaterally to the ipsilateral ear through a TDH 49 headphone (Telephonics, New York, USA) while the volunteer sat on a desk with the head turned towards the contralateral side. The EMG signals were amplified (5k X), filtered (bandpass 10–2 kHz), and averaged (sweep time of 100 ms, 300 sweeps, 20 ms delay, and rate of 10/s). The n1-p1 inter amplitudes before and after vibration delivery were analyzed. MRI evaluation of endolymphatic hydrops: Gd-DTPA (500 mmol/L, Consun, Guangzhou, China) was administered through either intravenous injection (0.1 mmol/kg) or intratympanic injection (100 mmol/L, 0.5 mL). For the intratympanic injection, the defined amount of Gd-DTPA was delivered onto the medial wall of the middle ear cavity using a safe tympanic manipulation catheter (patent no. ZL 2013 2 0548546.1) after the tympanic membrane was perforated in the posterior-inferior quadrant using a 25-gauge spinal needle. Inner ear MRI measurements were performed at 4 h after an intravenous injection or at 12 h after an intratympanic injection of Gd-DTPA using a Tim 4G Head/Neck 20 coil (Siemens, Muenchen, Germany) on a 3 T machine (Skyra, Siemens, Muenchen, Germany). The inner ear images were obtained using a heavily T2-weighted 3-dimensional fluid-attenuated inversion recovery (hT2W-3D-FLAIR) sequence according to a previous reported protocol, with minor modifications [27]. Briefly, the following parameters were used: repetition time (TR): 9000 ms; echo time (TE): 544 ms; inversion time (TI): 2050 ms; rapid decrease to a constant (=0.5) flip angle of 120° for the turbo spin-echo refocusing echo train in the SPACE sequence; echo train length: 519; matrix: 324 X 384; 104 axial 1.0-mm-thick slices covering the labyrinth; FOV: 165 X 196 mm2; GRAPPA acceleration factor: 2; voxel size: 0.51 ×0.51×1.0 mm3; fat saturation: 1.0 mm; NEX 4; scan time: 15'11''. Statistics: The cVEMP amplitude on each side and amplitude side differences of MD post-vibration exposure were compared to that before vibration exposure using the Wilcoxon signed ranks test. The symptoms of vertigo, hearing loss, tinnitus, and ear fullness were scored as followings: disappeared=1, improved=2; remained the same=3, became worse=4; Severity of impact was rated and scores post-vibration were compared to that before vibration exposure using the Wilcoxon signed ranks test. A p-value of <0.05 was regarded as significant. | ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
| ||||||
|
Results | ||||||
|
Outcome of the MD patients after low-frequency vibration treatment: 12 out of 14 patients felt comfortable on the second day after exposure to low frequency vibration therapy. There were two patients without any benefit after the vibration. In the follow-up period vertigo disappeared in 4 out of 9 patients, occurred at a less intensity than before vibration in 3 out of 9 patients, and remained with the same intensity in 2 out of 9 patients. The low-frequency vibration was significantly effective in controlling the vertigo during the follow-up period (p<0.01, Wilcoxon signed ranks test). Hearing improved in 1 out of 9 patients after vibration therapy, while did not show any change in 8 out of 9 patients. The changes in hearing function were insignificant (p>0.05, Wilcoxon signed ranks test). Tinnitus disappeared in 1 out of 9 patients, appeared with a less intensity in 4 out of 10 patients, remained with the same intensity as before vibration in 4 out of 9 patients. The symptom of tinnitus was significantly improved by the low-frequency vibration (p<0.05, Wilcoxon signed ranks test). Ear fullness disappeared in 1 out of 9 patients, existed at a less intensity than before vibration in 4 out of 9 patients, remained with the same intensity as before vibration in 4 out of 9 patients. The symptom of ear fullness was significantly improved after the treatment (p>0.05, Wilcoxon signed ranks test) (Table 1). Changes in amplitudes of cVEMP of MD patients induced by low-frequency vibration: There was a tendency that the amplitudes of cVEMP at 30 min post-vibration were higher than that before vibration on the ipsilateral side though the changes were insignificant (p>0.05, Wilcoxon signed ranks test) (Table 2). The amplitudes of cVEMP at 30 min post-vibration were significantly lower than that before vibration on the contralateral side and consequently the side difference in the amplitudes of cVEMP (contralateral side minus ipsilateral side) became smaller at 30 min post-vibration than that before vibration (p<0.05, Wilcoxon signed ranks test) (Table 2). | ||||||
| ||||||
|
| ||||||
| ||||||
|
Discussion
| ||||||
|
The present preliminary work showed that application of the novel device for treating MD by delivering low-frequency vibrations to the mastoid process resulted in significant vertigo control and significant improvement in tinnitus and ear fullness during the observational period. Our results are in accordance with the report by Thomsen et al. that both endolymphatic-sac surgery and mastoidectomy are beneficial for MD in controlling symptoms of nausea, vomiting, vertigo, tinnitus, hearing impairment, and pressure in the ears [11] [12]. Saliba et al. recently reported that endolymphatic duct blockage is more effective than traditional endolymphatic sac decompression in controlling the symptoms of MD with a suspected mechanism of decreasing the volume of endolymph in the inner ear [28]. However, the reported surgery further favor our idea of vibration induced beneficial effects on MD patients although the potential blockage on backflow of harmful inflammatory cytokines from the endolymphatic sac into the inner ear by the procedure may provide additional therapeutic effect. The involvement of inflammation in the attacks of MD symptoms was supported by the study of Kitahara et al. showing super effects of endolymphatic sac drainage with intra-endolymphatic sac steroids in treating intractable MD [29]. The hypothesis for beneficial effect of the low-frequency vibrations on MD is that they induced shear stress in the inner ear. It is known that a tangential force acts on the surface of solid structures interfacing a flowing fluid, which is called a shear force. In the vascular bed, the fluid produces shear stress on the endothelium when blood is flowing in the artery. The nature of fluid flow in the vessel is dependent on the velocity of the flow and might be either laminar or oscillatory (turbulent). The gene expression pattern in the endothelial cells induced by a laminar flow was differentfrom that induced by oscillatory flow [30]. In the auditory system, sound waves are transformed via the mechanics of the middle ear from air-borne media to fluid flow in the cochlear compartments, and the organ of Corti converts the mechanical vibrations into sensory inputs. Vibration induced shear stress generated in the cochlea of guinea pigs up regulated the expression of tumor necrosis factor-α (TNF- α), vascular endothelium growth factor (VEGF), and TNF and VEGF receptors in the cochlea [15]. No hearing loss occurred in the normal subjects who were exposed to the present vibration system (data will be reported separately), indicating that the system was safe for the auditory system and the above cochlear shear stress was unlikely to be involved in the inner ear response of the novel system. In this study, the low-frequency vibration enhanced the amplitudes of cVEMP on the exposed side and suppressed that on the contralateral side. Finally, the side difference in the amplitudes of cVEMPs became smaller indicating a re-balanced vestibular response. The beneficial effect on vestibular system occurred by 12–24 h after delivery of the low-frequency vibrations, suggesting the involvement of biological responses rather than simple mechanical processes. The activity of the vestibular end organ in MD patients is complex. Manzari et al. observed the oVEMP and cVEMP following bone-conducted vibration in MD patients during quiescence vs. during acute attacks. These researchers found that during MD attacks, the dynamic utricular function in the affected ear (as measured based on the n10 wave of the oVEMP at 500 Hz) was enhanced, whereas the dynamic saccular function in the affected ear (as measured based on the p13 of the cVEMP following 500-Hz bone-conducted vibration) was reduced [23]. Using extracellular single-neuron recordings of primary vestibular neurons identified by their location and their responses to natural stimulation, Curthoys et al. found that there is a very clear preference for irregular otolith afferents to be selectively activated by bone-conducted vibrational stimuli at low stimulus levels and that bone-conducted vibrational stimuli activated some of the irregular utricular afferent neurons [31]. It is possible that the present vibratory system activated these elements in the vestibular end organs and established a new balance in both ears. Patients 4, 7, and 11 had obvious endolymphatic hydrops shown by MRI scan and gained relief of both the vestibular and cochlear symptoms that persisted for 7 m to 13 m. The low-frequency vibrations generated by the present novel device may have reset the excitability of various vestibular neurons and resulted in an adapted vestibular system rather than interfering with the endolymphatic hydrops. It could not be ruled out that the low-frequency vibrations adjusted the homeostasis of the inner ear by activating the transient receptor potential vanilloid (TRPV)-2 molecules in the vestibule and the TRPV-4 molecules in the cochlea [32] [33] . The mechanism of significant improvement in tinnitus and ear fullness after treatment of low-frequency vibration is unknown. However, facilitations in ossicular chain mechanics and auditory function have been indicated in normal individuals after exposure to the low-frequency vibration (data will be reported separately). A tendency of improvement in hearing loss in MD after treatment using the low-frequency vibration in the present study further supported the beneficial effect in the auditory function. The vibration may reduce the stiffness of the ossicular chain. In temporal bone the vibration at the large amplitude of very low frequency waves (<200 Hz) results in the skull vibrating as a rigid body producing a transitory type of motion as first proposed by Barany [34]. The ossicular chain has a different mass from the skull and transmission of vibration through tendons, via the tympanic membrane and oval window to ossicular chain will lead the ossicular chain to vibrate on their own resonant frequency and in this vein reduce stiffness of their ligaments and joints. Although the mechanism of developing tinnitus and ear fullness in MD in unknown, an improved auditory function may release these symptoms. There were some recognized limitations in the present study. First we could not organize the test in placebo controlled randomized manner due to the strong character of vibratory stimulus. We plan to circumvent this problem by evaluating the outcomes of the treatment results blinded. The patient sample was small and more cases should be included in the future study in order to confirm the beneficial effect. The follow-up period was short (7–19 m), and 5/14 patients were lost to follow-up. There was a possibility that the MD patients recovered spontaneously during the follow-up period. A large patient volume should be included in the future observation. | ||||||
|
Conclusion
| ||||||
|
In conclusion, the novel therapeutic system for vertigo control in Meniere's disease patients was effective in delivering low-frequency vibrations to the mastoid process and possibly inducing shear stress within the inner ear. The suspected therapeutic mechanism of this treatment is modification of the excitability of the vestibular neurons that is similar to resting the system. | ||||||
|
Acknowledgements
| ||||||
|
This study was supported by the National Natural Science Foundation of China (81170914/H1304) and the 1255 project of Changhai Hospital, Second Military Medical University, Shanghai, China. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions:
Jing Zou – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Rishunzi Peng – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Guiliang Zheng – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Qing Zhang – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Esko Toppila – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Hongliang Zheng – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Ilmari Pyykkö – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2015 Jing Zou et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|
|
About The Authors
| |||
| |||
| |||
| |||
| |||
| |||
| |||
| |||